Thatleftistqueer - Court









More Posts from Thatleftistqueer and Others








zhou rui *breathes*




96 line were actually born in 2012
i feed stray cats in my backyard because the cats in those warrior books died for my rights
Some Tips On Organic Chemistry
When you’re looking at two compounds and wondering how they may react, pay attention to the carbons - if they are bonded to a halogen or something more electronegative than them, then they have a partial positive charge and they’re going to want anything that will give them more electrons (a.k.a. a nucleophillic attack). If they’re bonded to hydrogen, they have a partial negative charge and they become your nucleophile, which will want to give those electrons to something that’s lacking them. Once you get these basics down, mechanisms become much easier to memorize because you can see the logic in them and sometimes predict them.
Get the basic mechanisms ingrained in your brain. Think of SN1, SN2, E1 and E2 as your new multiplication table. Make flashcards about them and take them to class. Or put them at the back or front of your notebook. Just have them handy at all times.
Draw the final steps in 3D. ALWAYS. You can draw the mechanisms and the first steps in 2D because it will make it easier to understand, but never forget that you’re working with a 3D structure that can flipped (and attacked by nucleophiles) every which way. Also, if you don’t know the basic perspectives used in orgo (Fischer, Newmans, sawhorse, wedge-dash) please take half an hour to learn them. Mainly wedge-dash and Fischer, but Newman is very useful when deciding which position you should put your atoms in if you’re dealing with sin and anti.
Colors. If you’re one of those people who ONLY writes in black pen, awesome, keep using it for WRITING. For reactions though, you’re going to want options. you’ll need to differentiate between:
The molecules (same color for atoms and bonds, unless you want to finish your notes on your deathbed).
Your three types of arrows: electron flow, actual steps in the reaction (think intermediates) and steps you may take to make it clearer for you but that happen at the same time.
Formal charges
The electrons that stay with its original atom and the ones that are given/shared, if you’re like me and you like your mechanisms to be spelled out.
This is not an excuse to go nuts with the coloring, 3 colors are enough. Personally, I use purple for molecules, electrons and reaction arrows, black for electron flow arrows and charges and light blue for clarification step arrows. Also optional but to denote a homolytic fission I usually write a blue line perpendicular to the bond. Similarly, if two atoms share one electron each, instead of just one them donating both electrons, I link said electrons with blue.
Remember to be consistent, otherwise you’ll end up like me, looking at your notes from the beginning of the semester and wondering if that dash is a bond or a -1 formal charge (to avoid this, preferably circle formal charges. Lol I never do this but I should).
Flashcards are so helpful! Write the reactants on one side and the mecanism and products on the other. Test yourself until you are one with the electrons.
If it’s a concerted mechanism, number the arrows. You’ll thank yourself a month from now.
Khanacademy. Khanacademy will save your butt when it comes to mechanisms. Chemwiki is likely to have anything that Khanacademy doesn’t. If it isn’t in either of those, Google images just became your new best friend. Books also tend to explain those nicely but I personally find them to be poorly structured and they usually include much more info than what you’ll actually be requiered to know. If you have the time to read two pages on a reaction though, by all means go for it.
Study in advance. Good luck studying for your final two days before if you don’t understand the mechanisms and you don’t have your material organized. Seriously, don’t do it. A week before the exam you could make those flashcards mentioned above. They’re a great way to review but it will be impossible if you are learning these things from scratch.
Get your hands on past tests. This goes for any subject but especially for orgo. Try to get a past test or at least ask an upper-classman who’s taken orgo with that professor. Does his/her tests focus on mechanisms? Retrosynthesis? Or does he/she give you the reactants and ask what the product is or what environment they should be in to obtain x? Ideally, you should be able to answer any of these if you know the material. However, if they focus on retrosynthesis, it may be a little tricky, so make sure to cater your study techniques to that.
You should also check out @colllegeruled’s Surviving Organic Chemistry, it’s super helpful and it has lots of resources (seriously, you introduced me to Khanacademy, I OWE YOU MY LIFE).
So, this is what I can offer so far. I hope it shines at least a faint light into the dark path that is organic chemistry.
Other masterposts
How To Stop Procrastinating
Memorization Tips
Skincare 101
Taking Notes in College
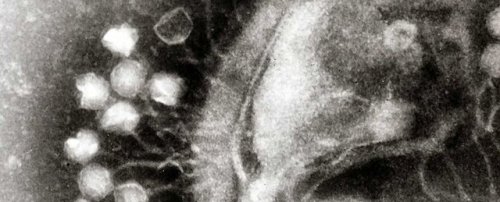
A century-old discovery of a virus could become our solution to antibiotic resistance
Antibiotic resistance - the phenomenon in which bacteria stop responding to certain antibiotics - is a growing threat around the world.
It’s expected to kill 10 million people annually by 2050.
And it hasn’t been easy to develop new drugs in order to stay ahead of the problem. Many major pharmaceutical companies have stopped developing new antibiotics, and the drugs that are still in development have faced numerous stumbling blocks toward approval.
So some drugmakers are starting to turn to other solutions, including one that’s actually had a fairly long history: phage therapy.
The treatments are made of bacteria-killing viruses called bacteriophages, or phages for short. Discovered in the early 1900s, bacteriophages have the potential to treat people with bacterial infections.
They’re commonly used in parts of eastern Europe and the former Soviet Union as another way to treat infections that could otherwise be treated by antibiotics. Because they are programmed to fight bacteria, phages don’t pose much of a threat to human safety on a larger scale.
“There’s huge potential there that regular antibiotics don’t have,” NYT columnist Carl Zimmer told Business Insider in 2015. “I think what we’d actually have to work on is how we approve medical treatments to make room for viruses that kill bacteria.”

Haunting Illustration of Lady Stoneheart by weremoon





call call call - choreography








types of fans during the fanchant, as demonstrated by seventeen
-
 aquiary liked this · 4 years ago
aquiary liked this · 4 years ago -
 soobissexual liked this · 4 years ago
soobissexual liked this · 4 years ago -
 mjh0 liked this · 4 years ago
mjh0 liked this · 4 years ago -
 keep-hauntingme liked this · 4 years ago
keep-hauntingme liked this · 4 years ago -
 sereiaxz reblogged this · 4 years ago
sereiaxz reblogged this · 4 years ago -
 ruivion liked this · 4 years ago
ruivion liked this · 4 years ago -
 munwoonie liked this · 5 years ago
munwoonie liked this · 5 years ago -
 phulaowa liked this · 5 years ago
phulaowa liked this · 5 years ago -
 dopilsdrum liked this · 5 years ago
dopilsdrum liked this · 5 years ago -
 celeste-ish liked this · 5 years ago
celeste-ish liked this · 5 years ago -
 flyawaywoo-blog liked this · 5 years ago
flyawaywoo-blog liked this · 5 years ago -
 sscarletvenus liked this · 5 years ago
sscarletvenus liked this · 5 years ago -
 moonlights-wolf liked this · 5 years ago
moonlights-wolf liked this · 5 years ago -
 lovleez reblogged this · 5 years ago
lovleez reblogged this · 5 years ago -
 angelshake liked this · 5 years ago
angelshake liked this · 5 years ago -
 bastianschweinsteigers liked this · 5 years ago
bastianschweinsteigers liked this · 5 years ago -
 passiveagressivegoldfish liked this · 5 years ago
passiveagressivegoldfish liked this · 5 years ago -
 cathartic-oh liked this · 5 years ago
cathartic-oh liked this · 5 years ago -
 sugahoneyss-blog liked this · 5 years ago
sugahoneyss-blog liked this · 5 years ago -
 dprmito liked this · 5 years ago
dprmito liked this · 5 years ago -
 jooncheeksquisher-blog liked this · 5 years ago
jooncheeksquisher-blog liked this · 5 years ago -
 imilkeanu liked this · 5 years ago
imilkeanu liked this · 5 years ago -
 makkuszone liked this · 5 years ago
makkuszone liked this · 5 years ago -
 cixsuki liked this · 5 years ago
cixsuki liked this · 5 years ago -
 busansblog liked this · 5 years ago
busansblog liked this · 5 years ago -
 obitu6rie liked this · 5 years ago
obitu6rie liked this · 5 years ago -
 comerhacefeliz liked this · 5 years ago
comerhacefeliz liked this · 5 years ago -
 xhakikyun liked this · 6 years ago
xhakikyun liked this · 6 years ago -
 dioramasmuse reblogged this · 6 years ago
dioramasmuse reblogged this · 6 years ago -
 dioramasmuse liked this · 6 years ago
dioramasmuse liked this · 6 years ago -
 gohaeg liked this · 6 years ago
gohaeg liked this · 6 years ago -
 daycarat liked this · 6 years ago
daycarat liked this · 6 years ago -
 gothicjeon liked this · 6 years ago
gothicjeon liked this · 6 years ago -
 wafflecitu liked this · 6 years ago
wafflecitu liked this · 6 years ago -
 king-of-being-forgotten liked this · 6 years ago
king-of-being-forgotten liked this · 6 years ago -
 precious--bby liked this · 6 years ago
precious--bby liked this · 6 years ago -
 gyullberry liked this · 6 years ago
gyullberry liked this · 6 years ago -
 eliotgiovanna-pv liked this · 6 years ago
eliotgiovanna-pv liked this · 6 years ago -
 luvsjohnny liked this · 6 years ago
luvsjohnny liked this · 6 years ago -
 ohmyena liked this · 6 years ago
ohmyena liked this · 6 years ago -
 fooolsun reblogged this · 6 years ago
fooolsun reblogged this · 6 years ago