Recently When Im Tempted To Say 'i'm Gonna Kill Myself' I Try To Correct It Into Saying "im Gonna Walk
recently when im tempted to say 'i'm gonna kill myself' i try to correct it into saying "im gonna walk into the river and become a trout" or some other form of that. this is my new thing
More Posts from Essentiallyless and Others
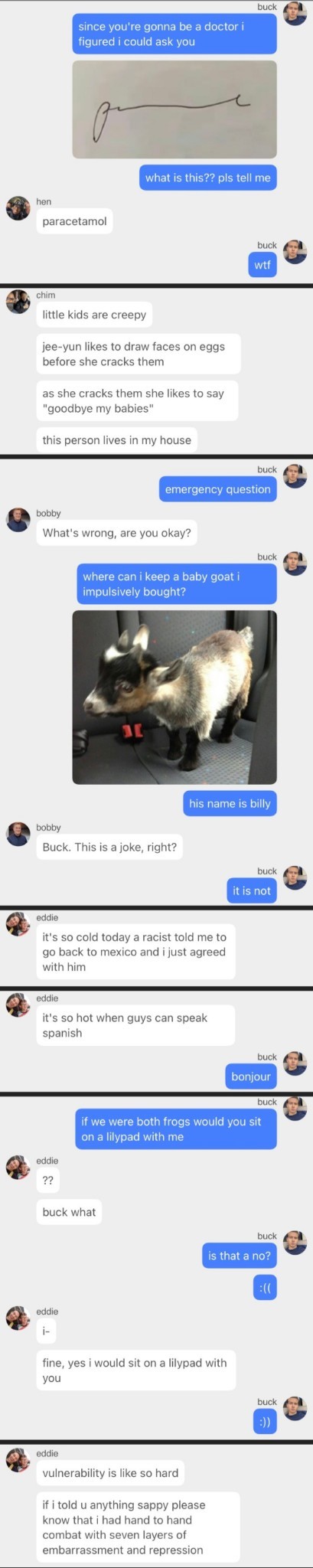
They call me bush on account of my w George
they call me George on account of my W Bush
Every hand that rises against Internet Archive shall fall
touching grass is not enough i need to be absolutely fucking railed in the nastiest way possible
sometimes i have a hard time wrapping my head around the existence of doms lol... like u wanna to do all of the thinking FOR ME??? u wanna take care of ME??? u wanna make ALL of the decisions??? u just need me to sit pretty and make you feel good???? literally what are you, an angel???????
michael romance. ray toro sighting. two gerard sightings in a week. she has gray hairs. michael is doing things. frank is posting. rehearsal. we are so fucking back

OKAY THIS ARTICLE IS SO COOL
I'm going to try to explain this in a comprehensible way, because honestly it's wild to wrap your head around even for me, who has a degree in chemistry. But bear with me.
Okay, so. Solids, right? They are rigid enough to hold their shape, but aside from that they are quite variable. Some solids are hard, others are soft, some are brittle or rubbery or malleable. So what determines these qualities? And what creates the rigid structure that makes a solid a solid? Most people would tell you that it depends on the atoms that make up the solid, and the bonds between those atoms. Rubber is flexible because of the polymers it's made of, steel is strong because of the metallic bonds between its atoms. And this applies to all solids. Or so everybody thought.
A paper published in the journal Nature has discovered that biological materials such as wood, fungi, cotton, hair, and anything else that can respond to the humidity in the environment may be composed of a new class of matter dubbed "hydration solids". That's because the rigidity and solidness of the materials doesn't actually come from the atoms and bonds, but from the water molecules hanging out in between.
So basically, try to imagine a hydration solid as a bunch of balloons taped together to form a giant cube, with the actual balloon part representing the atoms and bonds of the material, and the air filling the balloons as the water in the pores of the solid. What makes this "solid" cube shaped? It's not because of the rubber at all, but the air inside. If you took out all the air from inside the balloons, the structure wouldn't be able to hold its shape.
Ozger Sahin, one of the paper's authors, said
"When we take a walk in the woods, we think of the trees and plants around us as typical solids. This research shows that we should really think of those trees and plants as towers of water holding sugars and proteins in place. It's really water's world."
And the great thing about this discovery (and one of the reasons to support its validity) is that thinking about hydration solids this way makes the math so so so much easier. Before this, if you wanted to calculate how water interacts with organic matter, you would need advanced computer simulations. Now, there are simple equations that you can do in your head. Being able to calculate a material's properties using basic physics principles is a really big deal, because so far we have only been able to do that with gasses (PV=nRT anyone?). Expanding that to a group that encompasses 50-90% of the biological world around us is huge.
bro I’ve been abandoned so much it’s not even a fear at this point it’s just expected

this has reduced me to tears
-
 purplerayneyskyye reblogged this · 6 days ago
purplerayneyskyye reblogged this · 6 days ago -
 purplerayneyskyye liked this · 6 days ago
purplerayneyskyye liked this · 6 days ago -
 jfk-blown-away-blog reblogged this · 6 days ago
jfk-blown-away-blog reblogged this · 6 days ago -
 jfk-blown-away-blog liked this · 6 days ago
jfk-blown-away-blog liked this · 6 days ago -
 sailorsleepymoon reblogged this · 6 days ago
sailorsleepymoon reblogged this · 6 days ago -
 zelderina reblogged this · 6 days ago
zelderina reblogged this · 6 days ago -
 lovingmesomeme liked this · 6 days ago
lovingmesomeme liked this · 6 days ago -
 shanklinthestabpossum reblogged this · 6 days ago
shanklinthestabpossum reblogged this · 6 days ago -
 dognotman reblogged this · 6 days ago
dognotman reblogged this · 6 days ago -
 junipersdragon liked this · 6 days ago
junipersdragon liked this · 6 days ago -
 asleepinthestorm reblogged this · 6 days ago
asleepinthestorm reblogged this · 6 days ago -
 purple-hel reblogged this · 6 days ago
purple-hel reblogged this · 6 days ago -
 idontgiveadameron reblogged this · 1 week ago
idontgiveadameron reblogged this · 1 week ago -
 moonshine-aqua reblogged this · 1 week ago
moonshine-aqua reblogged this · 1 week ago -
 jeixart liked this · 1 week ago
jeixart liked this · 1 week ago -
 hostmodem reblogged this · 1 week ago
hostmodem reblogged this · 1 week ago -
 hostmodem liked this · 1 week ago
hostmodem liked this · 1 week ago -
 stellarseeming reblogged this · 1 week ago
stellarseeming reblogged this · 1 week ago -
 thegenderfluidace reblogged this · 1 week ago
thegenderfluidace reblogged this · 1 week ago -
 thegenderfluidace liked this · 1 week ago
thegenderfluidace liked this · 1 week ago -
 starlockfallsnomore reblogged this · 1 week ago
starlockfallsnomore reblogged this · 1 week ago -
 tenthirteenpm liked this · 1 week ago
tenthirteenpm liked this · 1 week ago -
 robots-and-sideblogs reblogged this · 1 week ago
robots-and-sideblogs reblogged this · 1 week ago -
 eepybogboy reblogged this · 1 week ago
eepybogboy reblogged this · 1 week ago -
 kuixotic reblogged this · 1 week ago
kuixotic reblogged this · 1 week ago -
 vox-imperium reblogged this · 1 week ago
vox-imperium reblogged this · 1 week ago -
 vox-imperium liked this · 1 week ago
vox-imperium liked this · 1 week ago -
 schizoangelic liked this · 1 week ago
schizoangelic liked this · 1 week ago -
 siyuki1234 reblogged this · 1 week ago
siyuki1234 reblogged this · 1 week ago -
 eveshmeve liked this · 1 week ago
eveshmeve liked this · 1 week ago -
 genderfear liked this · 1 week ago
genderfear liked this · 1 week ago -
 moomoo-milked reblogged this · 1 week ago
moomoo-milked reblogged this · 1 week ago -
 stellarseeming liked this · 1 week ago
stellarseeming liked this · 1 week ago -
 penis-mysterious reblogged this · 1 week ago
penis-mysterious reblogged this · 1 week ago -
 whobblywhale123 liked this · 1 week ago
whobblywhale123 liked this · 1 week ago -
 hisname-isjeff liked this · 1 week ago
hisname-isjeff liked this · 1 week ago -
 chimkinbunnii reblogged this · 1 week ago
chimkinbunnii reblogged this · 1 week ago -
 chimkinbunnii liked this · 1 week ago
chimkinbunnii liked this · 1 week ago -
 tenaoss liked this · 1 week ago
tenaoss liked this · 1 week ago -
 thesilentdumb liked this · 1 week ago
thesilentdumb liked this · 1 week ago -
 themagistersbirthright reblogged this · 1 week ago
themagistersbirthright reblogged this · 1 week ago -
 0cannibunny0 liked this · 1 week ago
0cannibunny0 liked this · 1 week ago -
 dragonbat liked this · 1 week ago
dragonbat liked this · 1 week ago -
 m0rph1a reblogged this · 1 week ago
m0rph1a reblogged this · 1 week ago -
 m0rph1a liked this · 1 week ago
m0rph1a liked this · 1 week ago -
 moshieee liked this · 1 week ago
moshieee liked this · 1 week ago -
 orgack20 liked this · 1 week ago
orgack20 liked this · 1 week ago -
 chirimichi reblogged this · 1 week ago
chirimichi reblogged this · 1 week ago -
 chirimichi liked this · 1 week ago
chirimichi liked this · 1 week ago